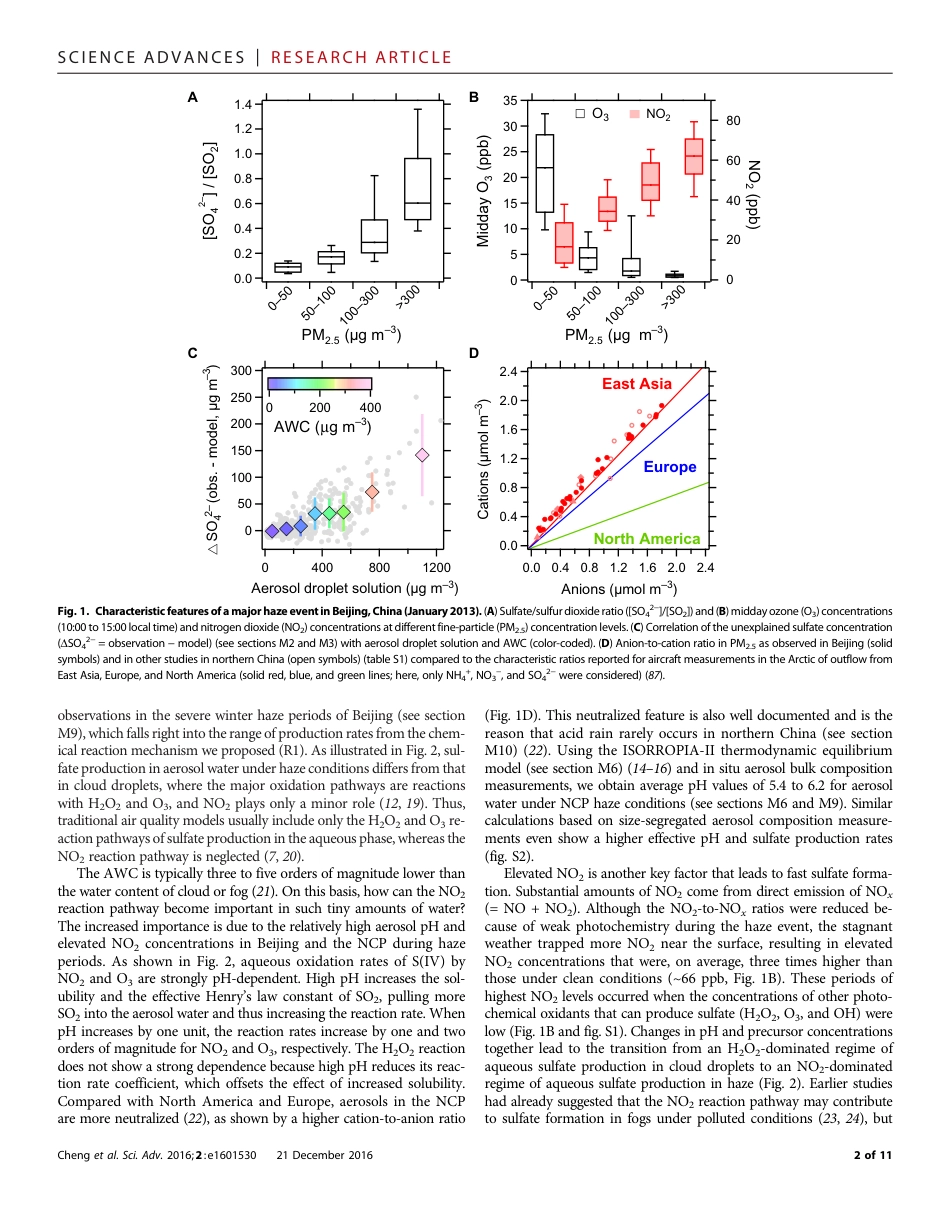
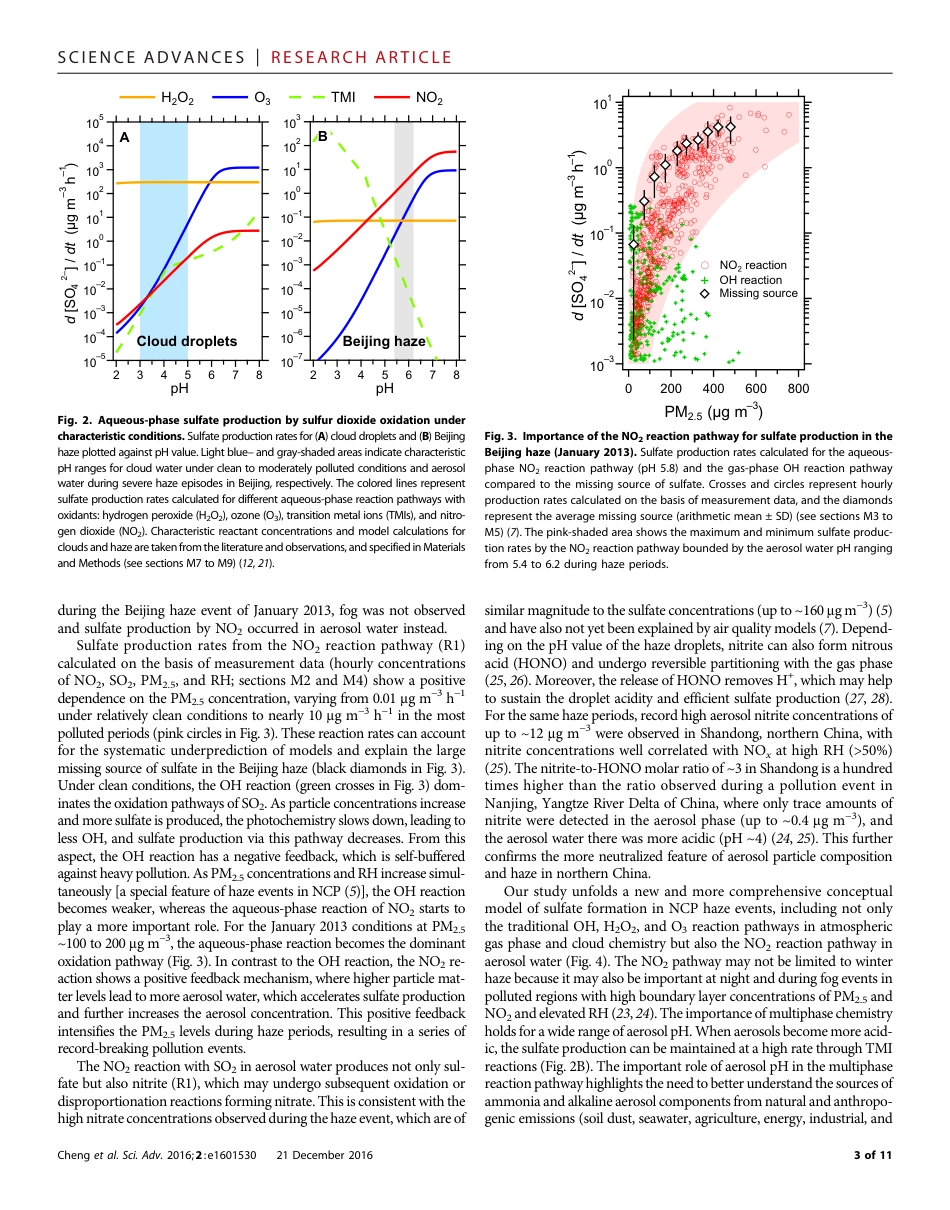
EN V I R O N M E N T AL S C I E N C E2016 © The Authors,some rights reserved;exclusive licenseeAmerican Associationfor the Advancementof Science. Distributedunder a CreativeCommons AttributionLicense 4.0 (CC BY).Reactive nitrogen chemistry in aerosol water as asource of sulfate during haze events in ChinaYafang Cheng,1*† Guangjie Zheng,1,2* Chao Wei,1 Qing Mu,1 Bo Zheng,2 Zhibin Wang,1Meng Gao,3,4 Qiang Zhang,5 Kebin He,2† Gregory Carmichael,3,4 Ulrich Pöschl,1† Hang Su6,1†Fine-particle pollution associated with winter haze threatens the health of more than 400 million people in the NorthChina Plain. Sulfate is a major component of fine haze particles. Record sulfate concentrations of up to ~300 mg m−3were observed during the January 2013 winter haze event in Beijing. State-of-the-art air quality models that rely onsulfate production mechanisms requiring photochemical oxidants cannot predict these high levels because of theweak photochemistry activity during haze events. We find that the missing source of sulfate and particulate mattercan be explained by reactive nitrogen chemistry in aerosol water. The aerosol water serves as a reactor, where thealkaline aerosol components trap SO2, which is oxidized by NO2 to form sulfate, whereby high reaction rates are sus-tained by the high neutralizing capacity of the atmosphere in northern China. This mechanism is self-amplifying be-cause higher aerosol mass concentration corresponds to higher aerosol water content, leading to faster sulfateproduction and more severe haze pollution.INTRODUCTIONPersistent haze shrouding Beijing and the North China Plain (NCP) dur-ing cold winter periods threatens the health of ~400 million people livingin a region of ~300,000 km2. Charact...